Faq
- What is titanium?
- Why can we see color from titanium?
- What is HORIE Corp's anodizing oxidation technology?
- What are the characteristics of titanium colors?
What is titanium?
For a very long period of time, oxidized titanium was used as white pigment for various applications. Titanium started to be utilized as a metal after the Second World War, and has built up a reputation of being lightweight, strong and corrosion-resistant. In comparison, titanium only weighs 2/3rds of a equal unit of steel, with similar strengths and also being corrosion-resistant.
Due to these characteristics, titanium has begun to be utilized quite frequently in the aeronautics industry, and in the construction of orbital satellites. And in recent times, titanium has also been accepted as being biocompatible; as in non-toxic to the body, and has begun to be utilized in the healthcare field and jewelry industry as well. Titanium is definitely a material which is being utilized and observed from many fields and industries in the world today.

Why can we see color from titanium?
Titanium is corrosion-resistant, but the surface of the material is covered with a very thin layer of naturally oxidized film (TiO2). This oxidized film is transparent and has a very strong refraction ratio. And when light hits this transparent film of oxidized titanium, the light is refracted and reflects back as if it's colored.
When we control the thickness of the oxidized titanium's transparent film by units of angstrom (10-8cms), then we are able to produce almost an unlimited number of colors. And as this transparent film has a high refraction ratio, it is also possible to produce glossy and bright colors.
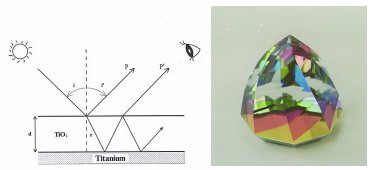
What is HORIE Corp's anodizing oxidation technology?
Very high technical achievement is required when controlling the thickness of oxidized titanium film. At HORIE Corporation, we have developed a very stabel and reliable anodizing oxidation treatment process.
In simple explanations, the anodized oxidation of titanium is achieved from the oxygen created from electrolysis. An oxidized titanium sheet is placed in a solution and anodized (+), and another conductive metal acts as a cathode (-). And with electrolysis, the oxygen formed on the surface of the titanium bonds with the titanium, creating a film of TiO2. [O2→(O)+2e-] In units of angstrom (10-8cms), the thickness of the TiO2 film is delicately controlled by slight changes in voltage and time.
To achieve gradation of color, such as the following rainbow like colors, the thickness of the TiO2 film is formed by continuously controlling the voltage during the electrolysis treatment while in a solution. This process is a completely different from printing technology which usually controls the size of dot printing sizes to control the distribution of the film thickenss.
What are the characteristics of titanium colors?
Depending on the light, morning, evening and even the angle in which the titanium suface is exposed to, the colored titanium may look slightly different. As the colored titanium utilizes the same color as the color spectrum, with different colored light, the colors may slightly alter.
We believe that the best light to observe the colored titanium surface is under a halogen lamp. Of course, holding your colored titanium product in your hand and viewing it from different angles is also a charm as you might notice some of the variations in color due to the angle of the exposed light.

